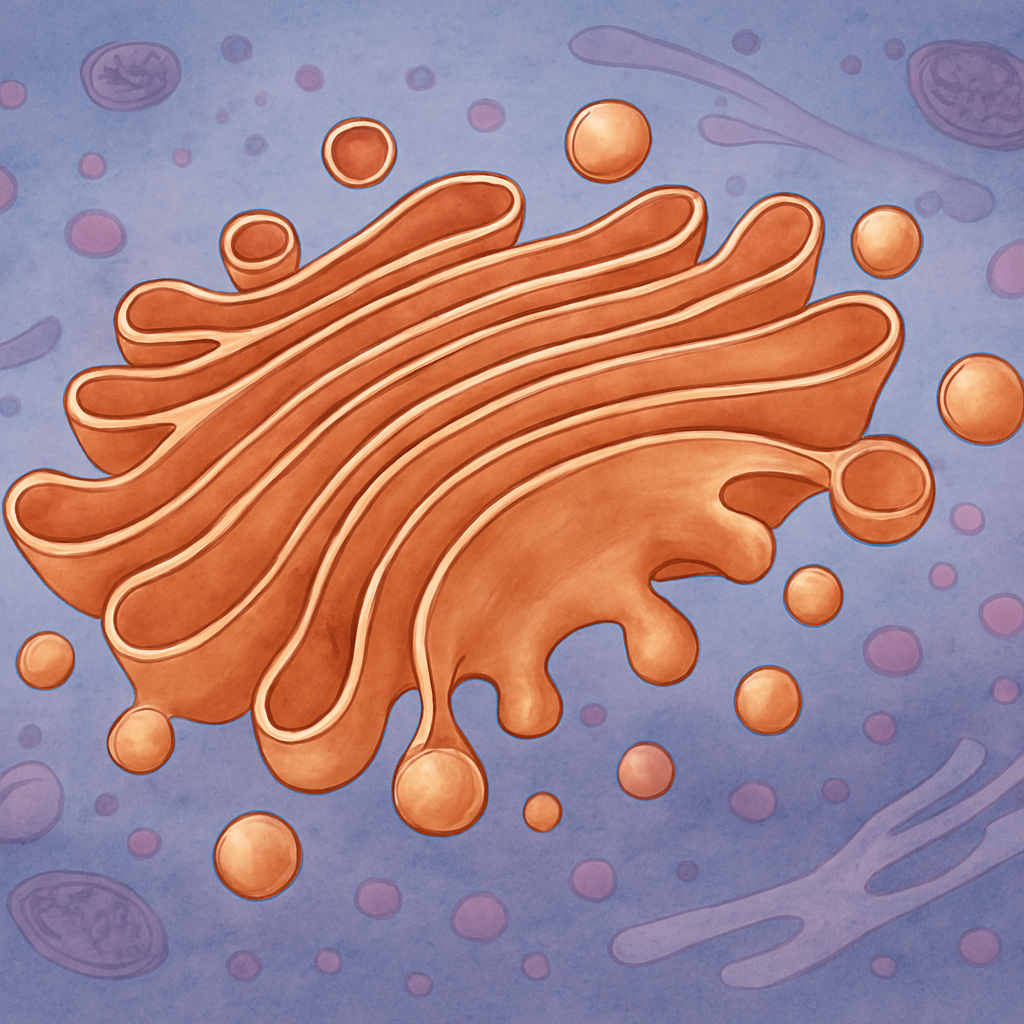
The Golgi apparatus, also known as the Golgi complex or Golgi body, is a crucial component of the cell’s internal structure, acting as the central sorting and packaging center for proteins and lipids.
In this article, we will delve into the intricacies of its structure, functions, and the roles it plays in disease and medicine.
Anatomy of the Golgi Apparatus
First discovered by Camillo Golgi in 1897, the Golgi apparatus is a series of flattened, stacked pouches called cisternae. The number of these stacks differs among various cell types and can range from just a few to several hundreds.
The Golgi apparatus is divided into three main regions: the cis-Golgi, medial-Golgi, and trans-Golgi, each corresponding to the entry, processing, and exit points of the apparatus. This structure allows for a stepwise modification and sorting of proteins and lipids that pass through it.
Functions of the Golgi Apparatus
Protein and Lipid Modification
The primary function of the Golgi apparatus is to modify, sort, and package proteins and lipids transported from the endoplasmic reticulum (ER). Proteins and lipids enter the cis-Golgi and undergo a series of modifications as they move through the cisternae to the trans-Golgi. Modifications include the addition or alteration of sugar chains (glycosylation), the removal of certain amino acids, and the addition of other biochemical functional groups.
Sorting and Packaging
The Golgi apparatus also plays a crucial role in sorting proteins and lipids for their correct destinations: within the cell (e.g., lysosomes), the plasma membrane, or outside the cell. Once sorted, these proteins and lipids are packaged into vesicles that bud off from the trans-Golgi.
Other Functions
The Golgi apparatus is also involved in the creation of lysosomes and plays a role in apoptosis (programmed cell death), cell signaling, and cell migration.
The Golgi Apparatus in Disease and Medicine
Defects in the Golgi apparatus can lead to various diseases. For example, alterations in Golgi function are associated with neurodegenerative diseases like Alzheimer’s and Parkinson’s. Furthermore, mutations affecting protein traffic through the Golgi apparatus can lead to congenital disorders of glycosylation.
Understanding the Golgi apparatus’s role in disease is crucial for the development of therapeutics. For instance, some cancer drugs target proteins that are modified and sorted by the Golgi apparatus, disrupting the cancer cells’ ability to proliferate.
Conclusion
The Golgi apparatus, while just one component of the cell, plays a pivotal role in cellular function and health. Understanding its complex structure and functions not only sheds light on the intricacies of cellular processes but also paves the way for potential medical advancements.
Test Your Knowledge on the Golgi Apparatus!
Answer the following questions to check your understanding of the article on this important cellular organelle.
Bibliography
Mironov AA, Beznoussenko GV. The Golgi Apparatus: State of the Art 110 Years after Camillo Golgi’s Discovery. Springer Science & Business Media; 2008. DOI: 10.1007/978-3-211-76310-0.
Rambourg A, Clermont Y. Three-dimensional electron microscopy: structure of the Golgi apparatus. Eur J Cell Biol. 1990;51(2):189-200. PMID: 2196165.
Boncompain G, Perez F. The Golgi apparatus in cell migration: an update. F1000Res. 2019;8. DOI: 10.12688/f1000research.18617.1.
Stanley P. Golgi glycosylation. Cold Spring Harb Perspect Biol. 2011;3(4):a005199. DOI: 10.1101/cshperspect.a005199.
Varki A. Biological roles of glycans. Glycobiology. 2017;27(1):3-49. DOI: 10.1093/glycob/cww086.
Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116(2):153-166. DOI: 10.1016/S0092-8674(03)01079-1.
Farquhar MG, Palade GE. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981;91(3 Pt 2):77s-103s. DOI: 10.1083/jcb.91.3.77s.
Joshi G, Chi Y, Huang Z, Wang Y. Aβ-induced Golgi fragmentation in Alzheimer’s disease enhances Aβ production. Proc Natl Acad Sci U S A. 2014;111(13):E1230-E1239. DOI: 10.1073/pnas.1320192111.
Freeze HH. Genetic defects in the human glycome. Nat Rev Genet. 2006;7(7):537-551. DOI: 10.1038/nrg1894.
Guo Q, Vasile E, Krieger M. Disruptions of Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by epsilon-COP. J Cell Biol. 1994;125(6):1213-1224. DOI: 10.1083/jcb.125.6.1213.